Collaboration
Partnership Model
In the collaboration model - the vaccine candidate development efforts will be managed collaboratively between the research partner(s) and MVDP through a joint development committee. Though MVDP will assume overall responsibility for advancement of vaccine candidate development. MVDP will utilize its broad capabilities and collaborations with numerous partners and stakeholders to support the development of the candidate malaria vaccines. It will be MVDP's responsibility to ensure collaboration between various partners through legal agreements defining scope of work, responsibilities and legal status of each partner.
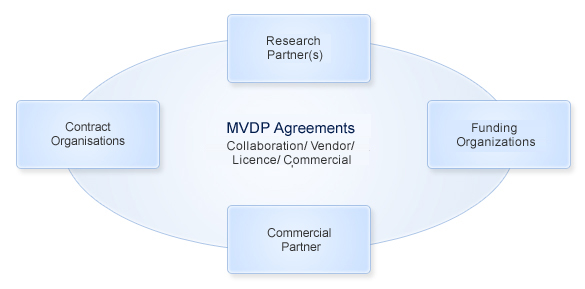
Research Partner(s): Will bring into the collaboration novel malaria vaccine antigen(s)/adjuvant/excepient/ platform technology/pre-clinical expertise
Funding Organizations: Will provide funds required for the execution of vaccine development program
Contract Organizations: Activities like cGMP manufacture, toxicology studies and clinical trials will be outsourced to appropriate vendors under a contract agreement with clearly defined IP assignment clause
Commercial Partner: A party who will be responsible for advancing the vaccine development beyond Phase IIb followed by licensure and commercialization on agreed terms. The commercial partner can be a second Research Partner
Agreements: Will be signed between different parties to define responsibilities, scope, IP and other relevant terms and conditions. These will be drafted and negotiated by MVDP on behalf of the research partner with other commercial and contractual partners
The critical discussion and decision points in a collaboration partnership are:
- Licensing of background IP rights of the research partner(s)
- Role played by the research partner(s)
- Securing and management of funds for vaccine development activities
- Timing the engagement of commercial partner
- Development of a mutually acceptable vaccine development plan
- Developing commercialization agreements with commercial partner






